
- 5 Core Quality Tools are the essentials of a quality management system in manufacturing.
- 5 Core Quality Tools consist of APQP, PPAP, FMEA, MSA, and SPC.
- Goal of 5 Core Quality Tools is to prevent errors rather than detect, creating impactful savings.
Table of Contents:
- What are the 5 Core Quality Tools?
- Why are the 5 Core Quality Tools important?
- 5 Core Quality Tools in Detail
- Future of 5 Core Quality Tools
What are the 5 Core Quality Tools?
The 5 Core Quality Tools are a set of sequential methods that form the essentials of a quality management system based on the IATF 16949 standard.
The focus is to eliminate or reduce potential errors early in the manufacturing process rather than later— prevention vs cure.
The 5 Core Quality Tools are comprised of:
- APQP (Advanced Product Quality Planning)
- PPAP (Production Part Approval Process)
- FMEA (Failure Mode and Effects Analysis)
- MSA (Measurement Systems Analysis)
- SPC (Statistical Process Control)
The 5 core tools are recognized as standard quality tools for the automotive industry by AIAG, although they are also used in other manufacturing sectors such as aerospace, defense, medical, and pharmaceutical.
Goal of 5 Core Quality Tools
- Establish streamlined communication across design and manufacturing.
- Catch potential errors earlier before it becomes a costly problem.
- Create reliable, better, and faster processes.
Why are the 5 Core Quality Tools important?
In the manufacturing industry, the cost of poor quality (COPQ) measures the financial cost associated with process and product failures.
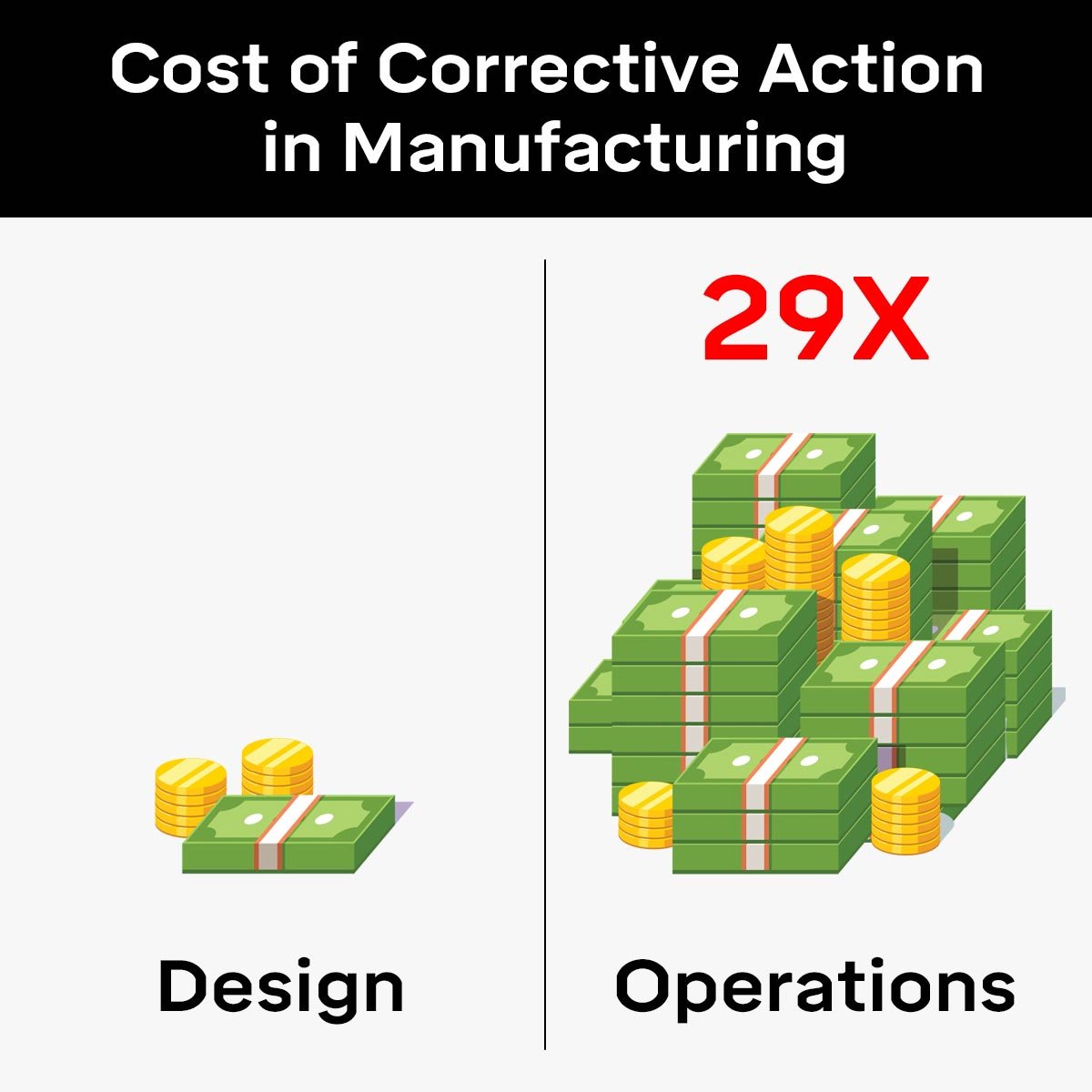
The best manufacturers have a COPQ score of around 1% whereas subpar manufacturers have a score of 5% or higher, which means subpar manufacturers are 5X more likely to pay in scrap, rework, defect, retesting, and recall costs compared to the leaders in the sector.
Therefore, the 5 Core Quality tools pre-emptively reduce COPQ by addressing problems, especially fatal flaws, before they become major problems after a product has been released. This is done by having better communication and understanding between the customer and supplier.
5 Core Quality Tools in Detail
Advanced Product Quality Planning (APQP)
APQP in one sentence: “Do we understand the expectations and have a structured planning process for it?”
APQP is the process framework for developing new products or processes specified by time-based milestones and defined inputs and outputs. This document aligns the manufacturer’s ability to meet customer requirements through standardized communication and reducing quality planning complexity.
The APQP process consists of 5 phases:
- Phase 1: Planning
- Phase 2: Product Design and Development
- Phase 3: Process Design and Development
- Phase 4: Product and Process Validation
- Phase 5: Feedback and Continuous Improvement
Learn More: APQP Explained: In-Depth Guide
Production Parts Approval Process (PPAP)
PPAP in one sentence: "Can we repeatedly and reliably make the part/product that meets customer expectations?”
PPAP is the formal process of documenting the supplier’s ability to understand the customer requirements, have agreed-to and documented expectations, and produce parts/products in a reliable and repeatable manner.
The PPAP report consists of 18 elements:
- Design Records: A copy of the drawing or model provided by customer. Design Records: A copy of the drawing or model provided by customer.
- Engineering Change Documents: Detailed description of changes of parts from previous revisions called Engineering Change Notice.
- Customer Engineering Approval: Customer approval of sample production parts.
- Design Failure Mode and Effects Analysis (DFMEA): Prediction of a product's potential design failure.
- Process Flow Diagrams: All steps in manufacturing process including components, measurement, and inspection.
- Process Failure Mode and Effects Analysis (PFMEA): Prediction of a potential process failure that could occur during production.
- Control Plan: Details the plan for how quality will be implemented to ensure a stable and reliable process.
- Measurement System Analysis (MSA): Conformance to customer’s ISO or TS standard. Usually Gage R&R for critical impact characteristics to control repeatability and reproducibility and confirmation that gages are calibrated to measure these characteristics to control measurement bias.
- Dimensional Results: A list of every dimension noted on the ballooned drawing or model with pass/fail assessment.
- Material / Performance Test Results: Summary of every test performed on the part, usually in the form of DVP&R (Design Verification Plan and Report).
- Initial Process Studies: Shows that critical processes are reliable. Includes SPC (statistical process control) charts.
- Qualified Laboratory Documentation: All industry certifications for validation testing.
- Appearance Approval Report (AAR): Customer approval on final product appearance including color, texture, fit, and more.
- Sample Product: Sample from initial production run.
- Master Sample: Sample part signed off by customer and supplier.
- Checking Aids: Detailed list of all tools used to inspect and measure parts.
- Records of Compliance with Customer-Specific Requirements: List of customer’s specific requirements for PPAP process.
- Part Submission Warrant (PSW): Summary of entire PPAP submission.
Learn More: PPAP Explained: In-Depth Guide

Need help understanding the 5 Core Quality Tools?Contact us today and our Support Team can helpContact Us
Failure Mode and Effects Analysis (FMEA)
FMEA in one sentence: “What can go wrong in the process or product, and how bad can it get?”
FMEA is a risk assessment method for evaluating potential modes of failure, the consequences and severity of those failures, and actions to prevent or mitigate those failures.
There are many types of FMEA including design (DFMEA), process (PFMEA), system, software, etc.
FMEA’s risk analysis is prioritized by 3 categories:
- Severity: How badly does the risk affect customers?
- Occurrence: How often will the risk happen?
- Detection: How easy is it to detect the risk?
Learn More: FMEA Explained: In-Depth Guide
Measurement Systems Analysis (MSA)
MSA in one sentence: “How do we know the tools we’re using for measuring are accurate?”
MSA is a statistical analysis of the reliability of the entire measurement system. It evaluates whether the variability in the measurement process is within acceptable tolerances and that any unacceptable tolerances may be due to equipment/gage, operator, method, environmental, etc. errors.
MSA is defined by 2 types of measurement variation:
1. Accuracy (close to target)
- Stability: accuracy over time.
- Linearity: accuracy throughout the measurement range.
- Resolution/Discrimination: accuracy with varying levels of detail.
- Bias: difference between observed value and reference value.
2. Precision (close to each other)
- Repeatability: Getting the same measurements using the same tool, same part, and same person.
- Reproducibility: Getting the same measurements using the same tool, same part, but different person.
Learn More: MSA Explained: In-Depth Guide
Statistical Process Control (SPC)
SPC in one sentence: “How do we know our process (people, tools, environment, etc.) is reliable?”
SPC is a statistical method of quality control that collects and analyzes variability data from product and process measurements—the goal being to determine what falls within process capability and corrective actions for what falls outside process capability.
Variation in manufacturing has two classes:
- Common cause variation: Variation that is normal and part of the standard process.
- Special cause variation: Variation that is abnormal and not part of the standard process.
The 14 most used SPC tools include:
Quality control tools:
- Cause-and-effect diagram
- Check sheet
- Control chart (most popular)
- Histograms
- Pareto chart
- Scatter diagram
- Stratification
Supplemental tools:
- Data stratification
- Defect map
- Event logs
- Process flowchart
- Progress center
- Randomization
- Sample size determination
Learn More: SPC Explained: In-Depth Guide
Future of 5 Core Quality Tools
Currently, most of the 5 Core Quality documentation is done manually through human transcription and interpretation.
Secondly, most 5 Core Quality data is used for pass/fail confirmation and discarded.
As digital transformation enters the manufacturing industry, connecting the digital thread (known as Industry 4.0) throughout the product lifecycle is vital for future growth.
Model-based definition and model-based enterprise are the processes of using an annotated 3D CAD model with manufacturing data (e.g. GD&T) that can make data collection for APQP, PPAP, FMEA, MSA and SPC more robust and faster, allowing automation, traceability, and compatibility across different silos.
More importantly, in the age of AI, Big Data, and deep learning, MBD provides the ability to data-mine the 5 Core Quality data for product and process improvements that can lead to breakthroughs and bottom-line impact.
Less reliance on manual processes. More futureproofing by digging to analytics. This is the future of manufacturing in a digital world.



